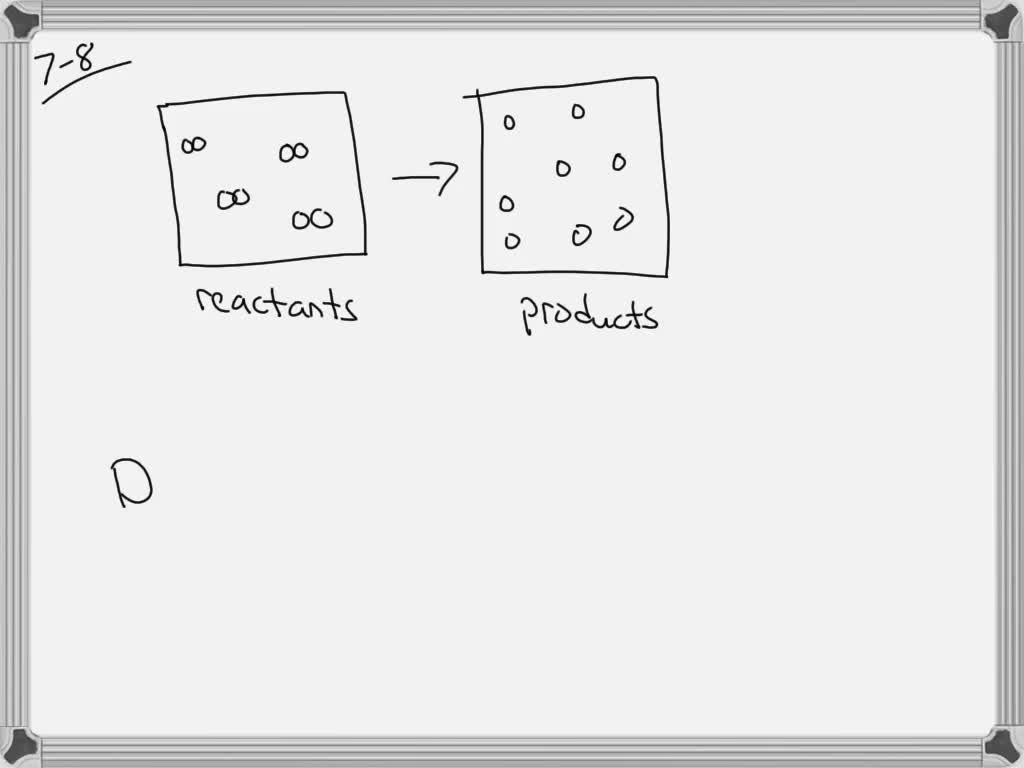
Reaction Vessel In Equilibrium . le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. State of a reaction mixture at which the forward reaction rate is equal to the reverse. As it applies to a chemical reaction system: The condition in a reaction when the concentrations of reactants and products cease to change. we can study this reversible reaction by placing hydrogen and iodine in a reaction vessel and then measuring the. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?.
from www.numerade.com
The condition in a reaction when the concentrations of reactants and products cease to change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. State of a reaction mixture at which the forward reaction rate is equal to the reverse. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of. what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. we can study this reversible reaction by placing hydrogen and iodine in a reaction vessel and then measuring the. As it applies to a chemical reaction system:
SOLVEDConsider the chemical reaction in the vessel depicted in the
Reaction Vessel In Equilibrium what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. The condition in a reaction when the concentrations of reactants and products cease to change. As it applies to a chemical reaction system: what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. we can study this reversible reaction by placing hydrogen and iodine in a reaction vessel and then measuring the. State of a reaction mixture at which the forward reaction rate is equal to the reverse. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in.
From www.bartleby.com
Answered A reaction vessel initially contains… bartleby Reaction Vessel In Equilibrium what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. State of a reaction mixture at which the forward reaction rate is equal to the reverse. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to. Reaction Vessel In Equilibrium.
From www.chegg.com
Solved Nitrogen and hydrogen react to form ammonia, like Reaction Vessel In Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. le cha telier's principle states that if a dynamic equilibrium is disturbed by. Reaction Vessel In Equilibrium.
From www.chegg.com
Solved A 100mL reaction vessel initially contains 2.60 x Reaction Vessel In Equilibrium we can study this reversible reaction by placing hydrogen and iodine in a reaction vessel and then measuring the. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. As it applies to a chemical reaction system: what property of a reaction can. Reaction Vessel In Equilibrium.
From www.chegg.com
Solved A reaction vessel contains an equilibrium mixture of Reaction Vessel In Equilibrium The condition in a reaction when the concentrations of reactants and products cease to change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. State of a reaction mixture at which the forward reaction rate is equal to the reverse. what property of. Reaction Vessel In Equilibrium.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free Reaction Vessel In Equilibrium chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. The condition in a reaction when the concentrations of reactants and products cease to change. le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of.. Reaction Vessel In Equilibrium.
From www.chegg.com
Solved A reaction vessel contains an equilibrium mixture of Reaction Vessel In Equilibrium State of a reaction mixture at which the forward reaction rate is equal to the reverse. The condition in a reaction when the concentrations of reactants and products cease to change. le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of. we can study this reversible reaction by placing. Reaction Vessel In Equilibrium.
From www.chegg.com
Solved Consider the equilibrium system described by the Reaction Vessel In Equilibrium The condition in a reaction when the concentrations of reactants and products cease to change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of.. Reaction Vessel In Equilibrium.
From www.bartleby.com
Answered Consider the equilibrium system… bartleby Reaction Vessel In Equilibrium we can study this reversible reaction by placing hydrogen and iodine in a reaction vessel and then measuring the. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. As it applies to a chemical reaction system: The condition in a reaction when the. Reaction Vessel In Equilibrium.
From www.numerade.com
Explain how decreasing the volume of the reaction vessel affects each Reaction Vessel In Equilibrium State of a reaction mixture at which the forward reaction rate is equal to the reverse. As it applies to a chemical reaction system: le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. The condition in a reaction when the concentrations of reactants and products cease to change.. Reaction Vessel In Equilibrium.
From www.youtube.com
13.8 g of N2O4 was placed in a 1 L reaction vessel at 400 K and allowed Reaction Vessel In Equilibrium le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. State of a reaction mixture at which the forward reaction rate is equal to the reverse. what property of a reaction can we use to predict the effect of a change in temperature on the value of an. Reaction Vessel In Equilibrium.
From www.chegg.com
Solved A reaction vessel contains an equilibrium mixture of Reaction Vessel In Equilibrium State of a reaction mixture at which the forward reaction rate is equal to the reverse. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with. Reaction Vessel In Equilibrium.
From studylib.net
Equilibrium for a general reaction Reaction Vessel In Equilibrium le cha telier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. we can study this reversible reaction by placing hydrogen and iodine in a reaction. Reaction Vessel In Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Reaction Vessel In Equilibrium what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products, and a reverse reaction, in. The condition in a reaction when the concentrations of reactants. Reaction Vessel In Equilibrium.
From www.youtube.com
At 700K, equilibrium constant for the reaction H2 (g) + I2 (g) ⇌ 2HI Reaction Vessel In Equilibrium what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. The condition in a reaction when the concentrations of reactants and products cease to change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products,. Reaction Vessel In Equilibrium.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Reaction Vessel In Equilibrium As it applies to a chemical reaction system: what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. State of a reaction mixture at which the forward reaction rate is equal to the reverse. The condition in a reaction when the concentrations of reactants and. Reaction Vessel In Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Reaction Vessel In Equilibrium what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. State of a reaction mixture at which the forward reaction rate is equal to the reverse. The condition in a reaction when the concentrations of reactants and products cease to change. le chatelier's principle. Reaction Vessel In Equilibrium.
From www.numerade.com
SOLVED An evacuated reaction vessel is filled with 0.800 atm of N₂ and Reaction Vessel In Equilibrium we can study this reversible reaction by placing hydrogen and iodine in a reaction vessel and then measuring the. what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. The condition in a reaction when the concentrations of reactants and products cease to change.. Reaction Vessel In Equilibrium.
From www.slideserve.com
PPT 10. Equilibrium State of a Gas Phase Reaction PowerPoint Reaction Vessel In Equilibrium what property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?. The condition in a reaction when the concentrations of reactants and products cease to change. chemical equilibrium is a dynamic process that consists of a forward reaction, in which reactants are converted to products,. Reaction Vessel In Equilibrium.